The quality of groundwater is determined by the initial quality of water infiltrating the subsurface, its interaction with the subsurface environment and the impact of anthropogenic activities at the surface (agriculture) or in the subsurface (e.g. oil and gas exploration). Therefore the ‘governing factors’ determining the potential threats to the quality of groundwater are the composition and reactivity of the subsurface strata (geogenic contamination) and contaminant sources from land use and other human activities (anthropogenic contamination). As a result, much like surface water, there may be multiple groundwater quality challenges at any given location.
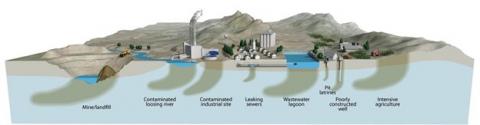
The groundwater environment differs significantly from surface water in ways that are important for the fate of natural and anthropogenic contaminants. It is dark and has no photosynthesis (but bioactivity exists, even though groundwater is aphotic), has a nearly constant temperature, has limited inputs from the surface (e.g. oxygen) and contains 102 to 106 times fewer bacterial organisms. The main source of natural groundwater recharge is precipitation. Most importantly, the groundwater zone has long water residence times, typically years to millennia compared to weeks for streams and rivers. This allows the groundwater time to react with rocks and minerals, which is important for reactions that are often slow. Some reactions, depending on mineralogy, may lead to geogenic contamination (As, Fe, Mn, F, radionuclides, etc.) but in other cases may facilitate natural attenuation of contaminants from the surface. The spatial scale of groundwater contamination largely depends on whether the contamination originates from point sources (e.g. factories) or diffuse sources of regional origin, for example of agricultural or atmospheric origin.
Several physical and chemical factors in groundwater may control processes and therefore the fate and mobility of contaminants.
Acidity is a key characteristic of groundwater. Acidity, measured as pH, in natural groundwater is controlled by the balance between carbonic acid (H2CO3) and buffering by dissolution of alkaline rocks. Besides controlling the precipitation and dissolution of minerals that may contain contaminants, the pH controls the mobility of a range of electrically charged contaminants by changing the surface charge of clays, oxides and organic matter (OM), solids whose surfaces promote sorption. This means that cationic contaminants like heavy metals (lead – Pb, zinc – Zn, cadmium – Cd, etc.) may be mobile at low pH values, while anionic contaminants, such as oxyanion forming elements (As, selenium – Se, etc.), may be mobile at neutral to high pH values. Similarly, organic contaminants may be adsorbed by naturally present organic matter, slowing the rate of contaminant transport in the groundwater (retardation).
The groundwater environment typically has low oxygen content because of slow, diffusion-controlled exchange with the atmosphere and because of the presence of natural organic matter in the groundwater aquifers, which consumes oxygen. The redox potential is a measure of the relative concentrations of dissolved oxidised and reduced species and is largely controlled by the balance of oxygen and labile organic matter. As for pH, the redox potential may indicate the degree of mobility for some groups of contaminants or the potential for natural attenuation of others. Typically, reducing conditions (i.e. high OM content) lead to an increase in dissolved Fe, Mn, hydrogen sulphide (H2S), As and ammonia (NH4). If dissolved sulphide is present, then a range of trace metal forming sulphide minerals may have very low mobility. Reducing conditions may also indicate a potential for the natural attenuation of nitrate and some organic contaminants.
High total dissolved solids (TDS but often measured as electrical conductivity EC) are associated with processes such as saltwater intrusion; dissolution of salts from highly soluble rocks and evaporites; high rates of evaporation in arid and semi-arid environments; or highly mineralised (old or deep) groundwater. High TDS are linked to high concentrations of major ions and sometimes geogenic contaminants (e.g. As, F, uranium – U). High TDS result in a high ionic strength and formation of soluble complexes that may lead to increased mobility for some ionic contaminants. High TDS is in itself a water quality issue.
Some water quality issues may result from a complex interplay of physical and inter-linked chemical processes. For instance, groundwater drawdown due to abstraction in rocks or sediments containing pyrite (FeS2) may lead to its exposure to the atmosphere and oxidation. In unbuffered environments the oxidation will cause acidification which in turn will lead to mobilisation of trace metals. The understanding of such linkages is a prerequisite for a sensible interpretation of international groundwater quality assessments.
Contaminants can be classified according to their origin in: