Numerous elements that dissolve from the minerals of the aquifer matrix under natural conditions and accumulate in groundwater can pose a potential health risk, as well as operational issues for water supply. These are known as geogenic contaminants. Two of the most widely documented geogenic contaminants are arsenic and fluoride, although others include iron, manganese, chromium and radionuclides such as uranium, radium and radon. If these naturally occurring groundwater contaminants are present in sufficiently high concentrations, they can lead to serious health problems, such as cancer (e.g. arsenic) or dental and skeletal problems (e.g. fluoride). Elevated iron and manganese concentrations (in association with microbiological action) commonly have aesthetic (orange, red and black staining of clothes and walls) and operational (clogging of boreholes, pumps and water reticulation infrastructure) impacts, the latter of which plays a critical factor in the success of groundwater supply systems and wellfields.
- Arsenic
-
In recent decades, arsenic (As) in groundwater supplies has become increasingly recognized as a major health issue. Although not an essential element for humans and animals, exposure often occurs through food, but most commonly through its natural presence in groundwater used for drinking. The health effects of consuming relatively low doses of arsenic over an extended period of time include disorders of the skin and vascular and nervous systems as well as various cancers.
Image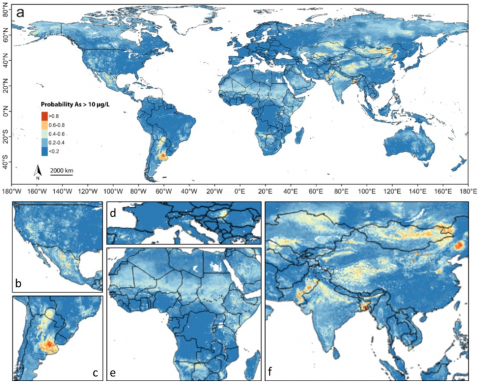
Probability of naturally occurring arsenic in groundwater Arsenic is naturally found in generally low concentrations in rocks all around the world. Under certain geochemical conditions, it can become mobilized in aquifers, particularly in river basins and deltas containing recently deposited sediments. Other geochemical settings leading to arsenic release include oxidation of arsenic-bearing sulphide minerals and release from arsenic-enriched geothermal deposits. The WHO guideline of 10 µg/L for arsenic in drinking water is exceeded on all continents, with hotspots including parts of Mexico, Argentina and South and Southeast Asia. The number of people estimated to be exposed to arsenic concentrations exceeding 10 µg/L for drinking and household uses is 94-220 million.
Groundwater with high concentrations of arsenic that is used for irrigation can directly increase arsenic levels in crops as well as negatively impact crop yields. This is particularly true for rice, which is very efficient in incorporating arsenic into its grains. Furthermore, irrigation with high-arsenic groundwater raises the level of arsenic in the topsoil, which can remain available for crop uptake long after ending irrigation with high-arsenic groundwater. The issue of arsenic exposure through the groundwater-crop pathway is particularly relevant in South and Southeast Asia, where there is extensive irrigation with arsenic-contaminated groundwater and much rice is produced and consumed.
- Fluoride
-
Image
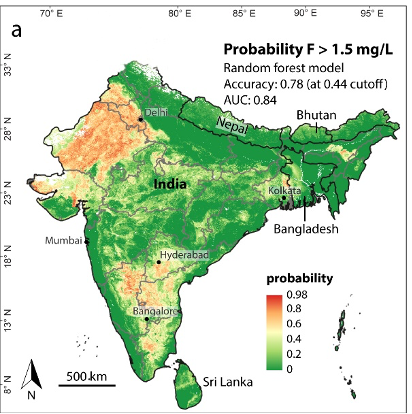
Exceeding the WHO guideline of 1.5 mg/L in India, Bangladesh, Bhutan, Nepal and Sri Lanka Fluoride (F-) is found in relative abundance in various minerals throughout Earth’s crust. It is widely present in groundwater as a result of geochemical interactions with fluoride-bearing minerals and the presence of geothermal fluids. Because of its small size and charge, fluoride is highly mobile in groundwater, and controlled by the availability of calcium and the pH of the water.
The main intake pathways of fluoride for humans are drinking water and food intake. Fluoride toxicity (fluorosis) occurs at higher levels of ingestion, which primarily consist of adverse effects on tooth enamel and skeletal tissue. In order to avoid excessive levels of fluoride, the WHO maintains a guideline for fluoride in drinking water of 1.5 mg/L. However, some countries, particularly in warmer climates, recommend a lower limit of 1.0 mg/L because of higher water consumption. Hotspots of groundwater fluoride include India, Mexico and the East Africa Rift System. It is estimated that 9% of the Indian population (120 million people) is potentially exposed to fluoride concentrations exceeding 1.5 mg/L, whereas the total population in the East African Rift affected by fluoride reaches 80 million, with more than 13 million people in Ethiopia living in high fluoride risk areas.
Image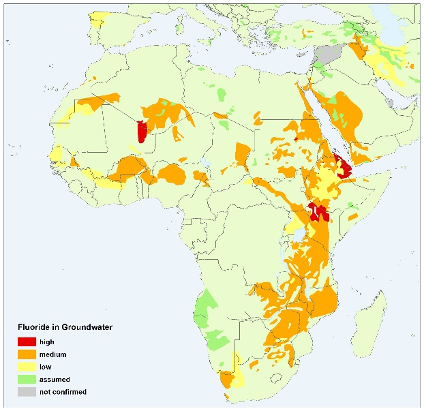
Africa (IGRAC, 2004) - Iron and manganese
-
Image

photos Umvoto Africa Iron (Fe) and manganese (Mn) are two of the most abundant metals in the Earth’s crust, and usually occur in association. Both elements are present in a range of rock forming minerals in igneous/metamorphic rocks and associated derived sediments and sedimentary rocks. Fe/Mn can also be introduced into various hard rock lithologies via hydrothermal oxide mineralisation in fractured zones, combined with later secondary supergene enrichment by groundwater flowing along preferential fracture paths. The form and solubility of Fe/Mn in groundwater is strongly dependent on the pH and redox potential of groundwater with Fe/Mn being mobile in either acidic or anaerobic groundwaters, with dissolved oxygen, dissolved organic carbon (and associated organic compounds such as humic, fluvic and tannic acids), salinity, sulphur and/or carbonate species also acting as controlling parameters.
Elevated Fe/Mn concentrations (usually above ~0.3 mg/L and ~0.1 mg/L respectively) can have a range of aesthetic and operational issues (with associated high investment, management and operation costs), if not removed through some form of in-situ borehole or post-abstraction groundwater treatment. Aesthetic problems from elevated Fe/Mn includes changes in the colour and turbidity of water (with an associated, unpleasant metallic taste), and the orange/black staining of laundry clothes and walls (following washing/irrigation and exposure to atmospheric oxygen). Most importantly from an operational aspect, elevated Fe/Mn can cause clogging/blockages of boreholes and associated aquifer matrix/fractures (reducing borehole/aquifer yield), as well as water/sanitation/irrigation reticulation infrastructure. This clogging is a result of the development of Fe/Mn oxide/hydroxide precipitation (due to oxygen ingress into the borehole during pumping) and associated bacterial sludge (via biofouling i.e. accumulation of Fe/Mn bacteria biofilms, which can also cause microbial-induced corrosion). Biofouling of boreholes, pumps and water reticulation infrastructure requires expensive periodic cleaning in order to ensure the continued functionality, operability and viability of groundwater abstraction systems.
There are no immediate health risks of elevated Fe in drinking water (WHO has no Fe drinking water guideline, although some countries e.g. South Africa have chronic health limits of <2 mg/L for Fe). Toxic symptoms are only observed after massive intake e.g. Fe concentrations of ~10-30 mg/L can have chronic health effects in young children and sensitive adults such as haemochromatosis (where tissue damage occurs as a consequence of Fe accumulation). Long term health impacts are increasing at Fe concentrations of ~30-100 mg/L. Mn toxicity can potentially occur in humans, and the WHO drinking water guideline is <0.4 mg/L. Elevated Mn can cause respiratory (e.g. lung embolisms and bronchitis) and neurological (e.g. hallucinations, nerve damage and Parkinson’s disease) problems. Elevated Fe concentrations above 5 mg/l may cause foliar damage to plants due to Fe precipitation, whereas elevated Mn can be toxic to various plant types (with Mn concentration toxicity dependent on the plant species).
- Chromium
-
Another potentially hazardous but infrequently monitored geogenic contaminant is chromium (Cr), which is also found in localized anthropogenic contamination associated with industrial activities or mining. In natural settings, high chromium concentrations are found predominantly in mafic aquifers, with mobility being influenced by pH. Geogenic chromium has been reported in aquifers in Europe and North and South America. Although an essential element, high doses of chromium can possibly be carcinogenic, thus the WHO has a set a provisional guideline value of 50 µg/L.
- Radioactive substances
-
Image
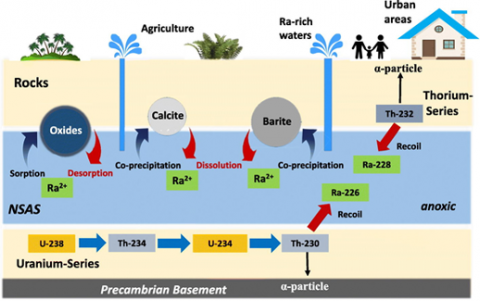
Origin of Ra-isotopes and mobilization mechanism in groundwater of the Sinai peninsula Rock and soil contain trace amounts of naturally occurring radioactive substances that can accumulate in groundwater and negatively affect its utilization. Most relevant natural radionuclides of concern for water supply are the water-soluble products of the uranium and thorium radioactive series of uranium (238U, 234U), radium (228Ra, 226Ra) and radon (222Rn). However, individual cases of other radionuclide anomalies in groundwater such as highly toxic polonium (210Po) have also been reported. Due to its short half-life (t1/2) of 3.8 days and volatility, 222Radon might be of concern only when the time between groundwater extraction and its use is short.
Dissolved uranium is often present in groundwater because of its moderate mobility, long half-life and relative abundance in the earth’s crust. The chemotoxicity of uranium is generally more significant than its radiotoxicity. However, in the presence of other radionuclides its contribution to gross activity concentrations might result in an excess of screening or guidance levels. Uranium concentrations exceeding the WHO guideline (30 µg/L) for drinking water have been found all over the world and are generally observed for oxic groundwater. Uranium concentrations strongly correlate with calcium and carbonate. The formation of uranyl carbonate complexes may allow uranium to be mobile at concentrations over 1 mg/L. Elevated dissolved uranium concentrations may originate from ore–grade deposits in sedimentary, granitic and volcanic host rocks, as well as uranium-enriched sedimentary facies associated with marine phosphorites that occur throughout North Africa and the Middle East.
The radium nuclide 226Ra is the fifth member of the 238U-decay series and the most abundant radium isotope in the environment in terms of mass due to its half-life of 1602 a. The second member of the 232Th-decay series is 228Ra (t1/2 5.8 a). Due to expensive and time-consuming radiochemical analysis, radium is generally not part of groundwater quality monitoring programmes. Nevertheless, a wide range of radium activity concentrations in groundwater has been reported worldwide. Anomalously high radium activities exceeding 10 Bq/L have been found in the United States, Europe, the Middle East, and Africa. Activity concentrations are generally related to uranium content in underlying sediments and bedrock and the geochemical environment. Dissolved radium is controlled by the availability of surface adsorption sites, which depends on the clay content and oxides in the aquifer rocks. The complex mechanisms resulting in radium mobilization and transport are not yet completely understood. The common assumptions that high dissolved radium occurs primarily in reduced, acidic, and/or saline groundwater is contradicted by observations in the Middle East where high dissolved radium concentrations occur in low-salinity, neutral-pH and oxygenated groundwater.